Introduction
When it comes to the fascinating world of chemistry, acids play a crucial role. They are known for their ability to donate protons, making them fundamental components in various chemical reactions. Acids vary in strength, with some being milder and others incredibly potent. In this article, we embark on a journey to unravel the mystery of the strongest acids known to science. From their unique properties to their practical applications, we’ll delve deep into the world of superacids.

The Hierarchy of Acid Strength
Understanding acid strength involves delving into the pH scale, a measurement system that quantifies the acidity or basicity of a substance. The pH scale ranges from 0 to 14, with lower values indicating stronger acids. At the extreme end of this scale lie the superacids, substances that go beyond the pH spectrum. These acids are not only rare but also incredibly powerful.
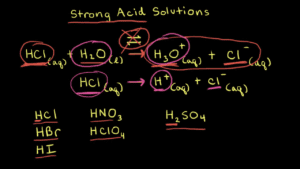
H2SO4 – Sulfuric Acid: The King of Acids
Sulfuric acid is often referred to as the “King of Acids.” With its molecular formula H2SO4, this acid is widely used in various industries, from battery manufacturing to fertilizers. Sulfuric acid is highly corrosive and has a low pH value, making it one of the strongest among common acids.
HF – Hydrofluoric Acid: The Silent Menace
Hydrofluoric acid, represented by the chemical formula HF, might not have the reputation of sulfuric acid, but it is exceptionally potent. What sets hydrofluoric acid apart is its ability to penetrate tissues, causing severe damage. It’s commonly used in industries such as glass etching and electronics manufacturing.
The Realm of Superacids
While sulfuric acid and hydrofluoric acid are undoubtedly strong, the world of superacids takes acidity to a whole new level. These acids can even protonate hydrocarbons, a feat that traditional acids can’t achieve.
HSO3F – Magic Acid: A Superacid Icon
Magic acid, or fluorosulfuric acid (HSO3F), is a superacid renowned for its exceptional protonating abilities. It’s formed by mixing sulfuric acid with antimony pentafluoride. Magic acid can break down hydrocarbons and is valuable in various chemical reactions and processes.
CF3SO3H – Triflic Acid: Expanding Horizons
Triflic acid, also known as trifluoromethanesulfonic acid (CF3SO3H), is another prominent superacid. It’s highly stable and capable of catalyzing reactions that were once considered unfeasible. Triflic acid finds applications in fields ranging from pharmaceuticals to petrochemicals.
Applications and Impact
The strength of these acids opens doors to a myriad of applications across diverse industries. From fuel production to pharmaceutical synthesis, superacids are catalysts of innovation.
Conclusion
In the realm of chemistry, acid strength is a fascinating aspect that showcases the diverse and powerful nature of these compounds. From the “King of Acids” sulfuric acid to the lesser-known superacids like magic acid and triflic acid, each substance offers unique properties and applications that drive scientific progress.
FAQs
Q: What determines the strength of an acid?
A: The strength of an acid is determined by its ability to donate protons.
Q: Are superacids dangerous to handle?
A: Yes, superacids are highly corrosive and require specialized handling procedures.
Q: Can superacids be found in nature?
A: Superacids are usually synthesized in laboratories and are not commonly found in nature.
Q: How does sulfuric acid impact the environment?
A: Improper disposal of sulfuric acid can lead to environmental damage due to its corrosive nature.
Q: What is the pH of hydrofluoric acid?
A: Hydrofluoric acid has a low pH value, making it highly acidic.