In the vast realm of chemistry, acids play a pivotal role in shaping the properties of various substances and reactions. While many of us are familiar with common acids like vinegar and lemon juice, there exists a fascinating and lesser-known category known as “superacids.” These extraordinary compounds exhibit an astonishing level of acidity, surpassing even the conventional strong acids that we encounter in our daily lives. In this article, we will delve into the captivating world of superacids, exploring their unique characteristics, applications, and the title question: “Which of the following is the strongest acid?”
Understanding Acidity: The Basics
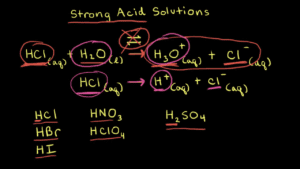
Before we can dive into the realm of superacids, let’s establish a fundamental understanding of acidity. Acids are substances that donate protons (H+ ions) to other substances, known as bases. This proton donation is what gives acids their characteristic sour taste and ability to turn blue litmus paper red. Acidity is measured on the pH scale, ranging from 0 to 14, with lower values indicating higher acidity.
The Concept of Superacidity
Superacids take the concept of acidity to an extreme level. These are not your everyday acidic compounds; they are substances that possess an acidity greater than 100%—an extraordinary feat given that traditional acids max out at 100% dissociation. One of the most renowned superacids is fluoroantimonic acid, HSbF6. This superacid is so potent that it can dissolve glass and even protonate hydrocarbons, transforming them into carbocations.

Comparing Superacids: Strength and Power
The race to determine the strongest superacid is a topic of continuous research and scientific curiosity. Among the contenders, fluoroantimonic acid often steals the spotlight due to its remarkable strength. However, other superacids like magic acid (a mixture of sulfuric acid and fluorosulfuric acid) and carborane superacids also boast formidable acidity levels. The true measure of their strength lies in their ability to stabilize the conjugate base after proton donation.
Applications of Superacids
Superacids might seem like exotic compounds with little practical use, but their unique properties have found applications in various scientific and industrial domains. One notable application is in the field of organic synthesis. Superacids can catalyze reactions that are otherwise challenging or impossible to achieve using traditional acids. They can facilitate the creation of complex molecules, thereby advancing fields like pharmaceuticals and materials science.
Challenges and Precautions
While superacids offer exciting possibilities, working with them requires extreme caution. Their unparalleled reactivity and corrosive nature demand specialized equipment and expertise. Researchers must handle these substances with the utmost care to prevent accidents and ensure their safety.
The Quest for Knowledge: Research and Discoveries
As technology and analytical techniques advance, scientists continue to uncover new insights into the world of superacids. The ongoing research seeks to not only identify even stronger superacids but also understand their behavior at the molecular level. These discoveries not only contribute to the scientific community’s understanding of chemical reactions but also pave the way for innovative applications in various industries.
Conclusion
In the captivating realm of chemistry, superacids stand as a testament to the remarkable diversity and complexity of chemical compounds. These potent substances challenge our conventional notions of acidity and offer a glimpse into the extraordinary capabilities of the molecular world. From fluoroantimonic acid to magic acid, the quest to determine the strongest acid showcases the relentless pursuit of knowledge and the boundless potential of human curiosity.
As we conclude this exploration, we’ve journeyed through the world of superacids, unraveling their unique characteristics, applications, and the ongoing scientific quest to identify the strongest acid. The chemistry of superacids is a testament to the intricate dance of protons and electrons, a dance that continues to inspire scientists and shape our understanding of the natural world.
Remember, while superacids represent a pinnacle of acidity, they are just one facet of the intricate mosaic that is chemistry. And as we step away from the world of superacids, we are reminded that the universe of science is vast, endlessly intriguing, and always ready to surprise us with its hidden wonders.


Pingback: Entry Level Surgical Tech Salary Guide: Average Earnings and Factors - Wise Financial Cents Blog